Polyelectrolytes
DEPL provides the best selection of polyelectrolytes suitable for the user based on the type of application. Our application engineers and laboratory have a diligent methodology for recommending the optimal polyelectrolyte and also the optimal dosing. We provide all 3 types of polyelectrolytes – Cationic, Anionic, and Non-Ionic
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are thus similar to both electrolytes (salts) and polymers (high molecular weight compounds) and are sometimes called Poly salts.
They are used to destabilize a colloidal suspension and to initiate flocculation (precipitation). They can also be used to impart a surface charge to neutral particles, enabling them to be dispersed in aqueous solution. They are thus often used as thickeners, emulsifiers, conditioners, clarifying agents, and even drag reducers.
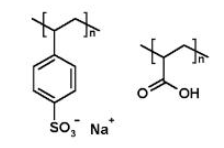
Chemical structures of two synthetic polyelectrolytes, as examples. To the left is poly(sodium styrene sulfonate) (PSS), and to the right is polyacrylic acid (PAA). Both are negatively charged polyelectrolytes when dissociated. PSS is a ‘strong’ polyelectrolyte (fully charged in solution), whereas PAA is ‘weak’ (partially charged).